|
The process of removing divalent cat
ions, usually calcium or magnesium, from water. When a sample of
water contains more than 120 mg of these ions per liter, expressed
in terms of calcium carbonate (CaCO3),
it is generally classified as
a hard water. Hard waters are frequently unsuitable for many
industrial and domestic purposes because of their soap-destroying
power and tendency to form scale in equipment such as boilers,
pipelines, and engine jackets. Therefore it is necessary to treat
the water either to remove or to alter the constituents for it to be
fit for the proposed use.
Water described as "hard" is high in
dissolved minerals, specifically calcium and magnesium. Hard water
is not a health risk, but a nuisance because of mineral buildup on
fixtures and poor soap and/or detergent performance. Hard water is

 |
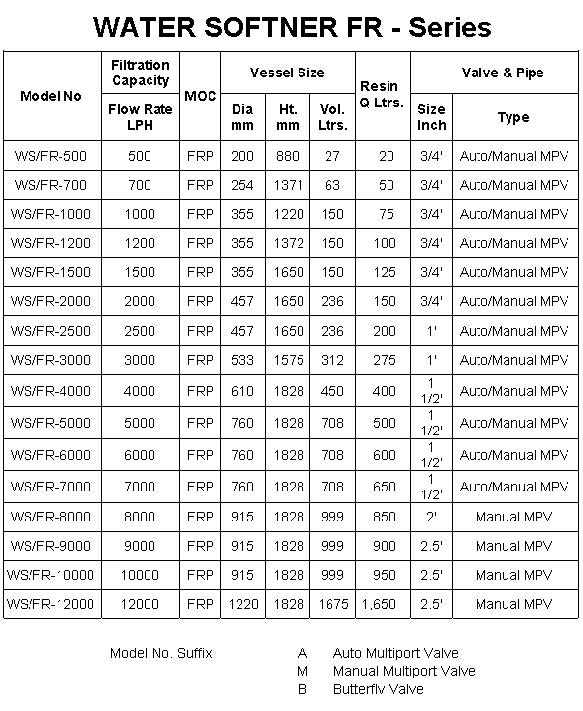
Water
Softener FR
Fiber Glass Reinforced Plastic (FGRP)
composite vessels are 1/3 the weight of carbon steel,
strengths are directly comparable to steel, no maintenance &
high aesthetic appearance.
• High performance Composite material
• Thermoplastic liner of polyester – wall thickness 3.8 mm
to 7.6 mm as per vessel diameter
• 100 % corrosion resistant
• Excellent bonding between inlet & PE liner
• Better curing at high temperature
• 200 mm to 1,000 mm diameter
• All are top opening & dia 450 mm onwards top &
bottom opening
• 450 mm onward flange fitting
• Operating Pressure – 10.5 kgf/cm2
• Operating temperature – 490 C
• 250,000 times cycle test from 10 psi to 150 psi
• Vessels are NSF & PED certified |
 |

 |
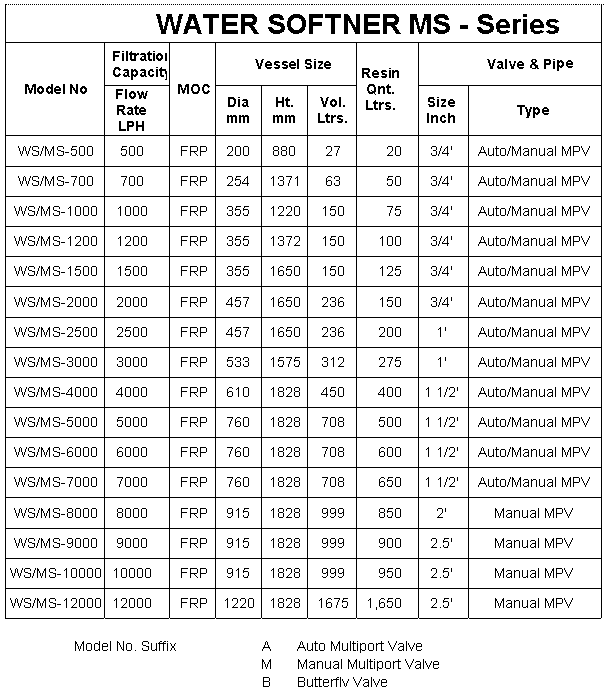
Water Softener MS
Mild Steel (MS) water softener are commonly used across the
world for following applications :
• Filter high quantity of water.
• Thermoelectric power plants.
• Boiler feed water
• Cooling tower. |
Sources of Hardness Minerals
In case of underground water, as water moves through soil and rock,
it dissolves very small amounts of minerals and holds them in
solution. Calcium and magnesium dissolved in water are the two most
common minerals that make water "hard." The degree of hardness
becomes greater as the calcium and magnesium content increases and
is related to the concentration of multivalent cations dissolved in
the water..
Indications of Hard Water
Hard water interferes with almost every cleaning task from
laundering and dishwashing to bathing and personal grooming. Clothes
laundered in hard water may look dingy and feel harsh and scratchy.
Dishes and glasses may be spotted when dry. Hard water may cause a
film on glass shower doors, shower walls, bathtubs, sinks, faucets,
etc. Hair washed in hard water may feel sticky and look dull. Water
flow may be reduced by deposits in pipes.
Dealing with hard water problems in the home can be a nuisance. The
amount of hardness minerals in water affects the amount of soap and
detergent necessary for cleaning. Soap used in hard water combines
with the minerals to form a sticky soap curd. Some synthetic
detergents are less effective in hard water because the active
ingredient is partially inactivated by hardness, even though it
stays dissolved. Bathing with soap in hard water leaves a film of
sticky soap curd on the skin. The film may prevent removal of soil
and bacteria. Soap curd interferes with the return of skin to its
normal, slightly acid condition, and may lead to irritation. Soap
curd on hair may make it dull, lifeless and difficult to manage.
When doing laundry in hard water, soap curds lodge in fabric during
washing to make fabric stiff and rough. Incomplete soil removal from
laundry causes graying of white fabric and the loss of brightness in
colors. A sour odor can develop in clothes. Continuous laundering in
hard water can shorten the life of clothes. In addition, soap curds
can deposit on dishes, bathtubs and showers, and all water fixtures.
Hard water also contributes to inefficient, costly operation and
maintenance of water-using appliances. Appliances like dish washing
machine, coffee & soft drink vending machines, ice cube machines use
very fine jets & delicate rubber parts in solenoids & pumps. These
jet nozzles gets checked & solenoids & pumps gets leakages problems
with hard water. Heated hard water forms a scale of calcium and
magnesium minerals that can contribute to the inefficient operation
or failure of water-using appliances. Pipes can become clogged with
scale that reduces water flow and ultimately requires pipe
replacement.
Interpreting Test Results
The hardness of your water will be reported in grains per gallon,
milligrams per liter (mg/l) or parts per million (ppm). One grain of
hardness equals 17.1 mg/l or ppm of hardness.
The Environmental Protection Agency (EPA) establishes standards for
drinking water which fall into two categories -- Primary Standards
and Secondary Standards.
Primary Standards are based on health considerations and Secondary
Standards are based on taste, odor, color, corrosivity, foaming, and
staining properties of water. There is no Primary or Secondary
standard for water hardness. Water hardness is classified by the
U.S. Department of Interior and the Water Quality Association as
follows:
|
Classification |
mg/l or ppm |
grains/gal |
|
Soft |
0 - 17.1 |
0 - 1 |
|
Slightly hard |
17.1 - 60 |
1 - 3.5 |
|
Moderately hard |
60 - 120 |
3.5 - 7.0 |
|
Hard |
120 - 180 |
7.0 - 10.5 |
|
Very Hard |
180 & over |
10.5 & over |
Water softening units can be permanently
installed into the plumbing system to continuously remove calcium
and magnesium. Water softeners operate on the ion exchange process.
In this process, water passes through a media bed, usually
sulfonated polystyrene beads. The beads are supersaturated with
sodium. The ion exchange process takes place as hard water passes
through the softening material. The hardness minerals attach
themselves to the resin beads while sodium on the resin beads is
released simultaneously into the water. When the resin becomes
saturated with calcium and magnesium, it must be recharged. The
recharging is done by passing a salt (brine) solution through the
resin. The sodium replaces the calcium and magnesium which are
discharged in the waste water. Hard water treated with an ion
exchange water softener has sodium added. According to the Water
Quality Association (WQA), the ion exchange softening process adds
sodium at the rate of about 8 mg/liter for each grain of hardness
removed per gallon of water.
For example , if the water has a hardness of 10 grains per gallon,
it will contain about 80 mg/liter of sodium after being softened in
an ion exchange water softener if all hardness minerals are removed.
Because of the sodium content of softened water, some individuals
may be advised by their physician, not to install water softeners,
to soften only hot water or to bypass the water softener with a cold
water line to provide un softened water for drinking and cooking;
usually to a separate faucet at the kitchen sink.
Softened water is not recommended for watering plants, lawns, and
gardens due to its sodium content.
Although not commonly used, potassium chloride can be used to create
the salt brine. In that case potassium rather than sodium is
exchanged with calcium and magnesium.
Before selecting a mechanical water softener, test water for
hardness and iron content . When selecting a water softener, the
regeneration control system, the hardness removal capacity and the
iron limitations are three important elements to consider.
There are three common regeneration control systems. These include a
time-clock control (you program the clock to regenerate on a fixed
schedule); water meter control (regenerates after a fixed amount of
water has passed through the softener); and hardness sensor control
(sensor detects hardness of the water leaving the unit, and signals
softener when regeneration is needed).
Hardness removal capacity, between regenerations, will vary with
units. Softeners with small capacities must regenerate more often.
Your daily softening need depends on the amount of water used daily
in your household and the hardness of your water. To determine your
daily hardness removal need, multiply daily household water use
(measured in gallons) by the hardness of the water (measured in
grains per gallon).
Example: 400 gallons used per day X 15 grains per gallon hardness =
6,000 grains of hardness must be removed daily.
Iron removal limitations will vary with water softener units. If the
iron level in your water exceeds the maximum iron removal capacity
recommended by the manufacturer of the unit you are considering,
iron may foul the softener, eventually causing it to become plugged.
|





